Executive Overview
Table of Contents

AION builds closed-loop hardware to read and write bioelectric states in deep tissue using electromagnetic and acoustic fields—a control layer for cell reprogramming that chemistry and gene therapy fundamentally cannot provide.
What does this paradigm mean practically?
- Extends cell reprogramming beyond molecular limits.
- Enables non-invasive devices to be orders of magnitude more precise in space and time than drugs or gene therapies.
- Unlocks bioelectric algorithms for controlling proliferation, differentiation, and regeneration that are infeasible with chemistry.
This executive summary offers an early view of our platform and vision.
– Benjamin and RJ
TL;DR #
- Cell reprogramming (turning any cell back into a stem cell) could reverse aging, but molecular approaches face impossible search spaces. Billions are spent screening cocktails, yet the combination space is larger than what can be tested in a lifetime.
- Even if perfect molecular cocktails existed, delivery is a near impossible hurdle. Different tissues reprogram at different rates, so systemic delivery causes liver failure before neurons benefit.
- Bioelectricity is the necessary exit. It collapses the combinatorial search space into tunable field parameters and provides the spatiotemporal control via sub-centimeter precision and dynamic timing that molecular approaches fundementally cannot.
- The thymus is our initial target ($80B oncology market). FDA endpoints are organ size and T-cell counts, our approach addresses the core problem (immune decline) with measurable control.
Introduction #
We are fundamentally changing the way people approach intervening in biology. Our big-if-true is that if biology can be more effectively controlled with physics than via our understanding of molecular pathways, then we can use non-invasive techniques to bring about desired changes in various disease states that come about with age, and then eventually, aging itself.
How do we get there? But first, how did we get here?
Like many, we were enamored by the discovery that we could reprogram cells from a somatic (differentiated) state, and convert them back into a stem cell. In 2006, Yamanaka was researching this when he discovered that by increasing the concentration of four special proteins inside the cell, now referred to as Yamanaka factors, you could turn any cell type in the body back into a stem cell.1 This was groundbreaking at the time, because up until this point, the only places to get stem cells were from embryos and other ethically debatable outlets.
To those with an interest in longevity, Yamanaka’s discovery was also a proof of concept that, insofar as we define age at the level of an individual cell, that we could reverse it. We have been able to reprogram cells of various types into stem cells for nearly 20 years now. What progress have we made towards translating this for aging and what are the barriers standing in our way?
There are effectively three problems in translating this approach to something that would bring about an aging reversal outcome in the body:
- Efficiency: cell reprogramming is slow (taking weeks) and succeeds in only a small fraction of treated cells.
- Precision: we need to be able to turn the clock back on cells partially, not all way way to stem cells.
- Timing and delivery: since tissues reprogram at different rates, preventing organ failure requires precise control over where and when reprogramming occurs.
Billions of dollars are pouring into this space, funding longevity companies to identify a strategy to translate this finding to a therapy that would lead to a regenerative outcome in humans. Most of these players are still working on problems one and two without having figured out how they will even address three.
So with all this investment, what has been the progress after 20 years of work? To answer, we’ll start by explaining the state of the art. The thinking is that, of the multitude of possible protein factor combinations that could lead to a reprogramming outcome, there is a perfect cocktail out there to be found that will yield a higher stem cell output in experiments. Read any update from NewLimit and this thinking is put clearly on display.
In recent years, following a seminal Chinese study, researchers began exploring pure chemical approaches. The original group demonstrated that four sequential cocktails containing 27 total molecules could induce stem cells in culture over several weeks.2 This led many to double down on the idea that high throughput screening was the way, and that somewhere out there in chemical space is a protocol that will lead to an ideal, high stem cell yield reprogramming outcome, and furthermore, that there may eventually be a drug or series of drugs to be taken to reverse aging.
Billions have been spent pursuing these routes with the primary metric of success being how many tens of thousands of inputs have been screened in what period of time, indicating progress in churning through this space. If we take the net set of possible transcription factors or chemical combinations required to achieve anything here, how big is the space? The answer is staggering large and somewhere between completely unfeasible and physically impossible.3
So, here we see the limits of current approaches as it pertains to problem one. What about problems two and three? These are best explained at the same time.
The most successful partial reprogramming study to date was done in 2016 by the Ocampo lab.4 They genetically edited mice at the embryonic stage in order to create an on-off switch in the mice that would respond to a drug called doxycycline(dox); exposure to the drug would trigger the mice to express the Yamanaka factors on command. The most successful protocol featured a 2 days on, 5 days off schedule, and this led to a 33% median lifespan extension in progeroid mice (from 18 to 30 weeks).
The problem is that this gene circuit was all or nothing for every tissue in the body, and different cell types reprogram at different rates. As far as organ systems are concerned, the liver reprograms the fastest, then the pancreas, then the kidney and so on with cells like neurons and bones reprogramming the slowest. When persistently expressing these reprogramming factors, the liver may reprogram to a point where some liver cells start to become stem cells before cells like neurons start to see benefit at all, until eventually you get liver failure. 5
There are 2 takeaways here:
- This approach only works by editing the embryo at birth to have this special circuit. Sorry everyone already alive! and
- In order to scale this to something that works in every tissue type, much, much more complex gene circuit engineering would need to be figured out so that every different tissue type in the body has this on-off switch to be triggered by a different benign chemical.
Now set gene editing aside. In the event a perfect set of compounds that could reprogram cells partially was found, the same problem would occur because when consuming a drug orally, it will first go the the liver where it will be processed before diffusing to the rest of the body, leading to the same core issue: fast reprogramming tissue fails before others begin to see benefits. Not to mention the ability for the drug to cross the blood brain barrier to reprogram neurons at all and other issues like this for hard-to-reach cells in the body.
Enough talk about problems.
The point in communicating this background is to make it clear that achieving control over timing and delivery would be a paradigm-shifting breakthrough. At AION, we are strongly convinced that bioelectricity is the key to unlocking this.
Bioelectricity Background #
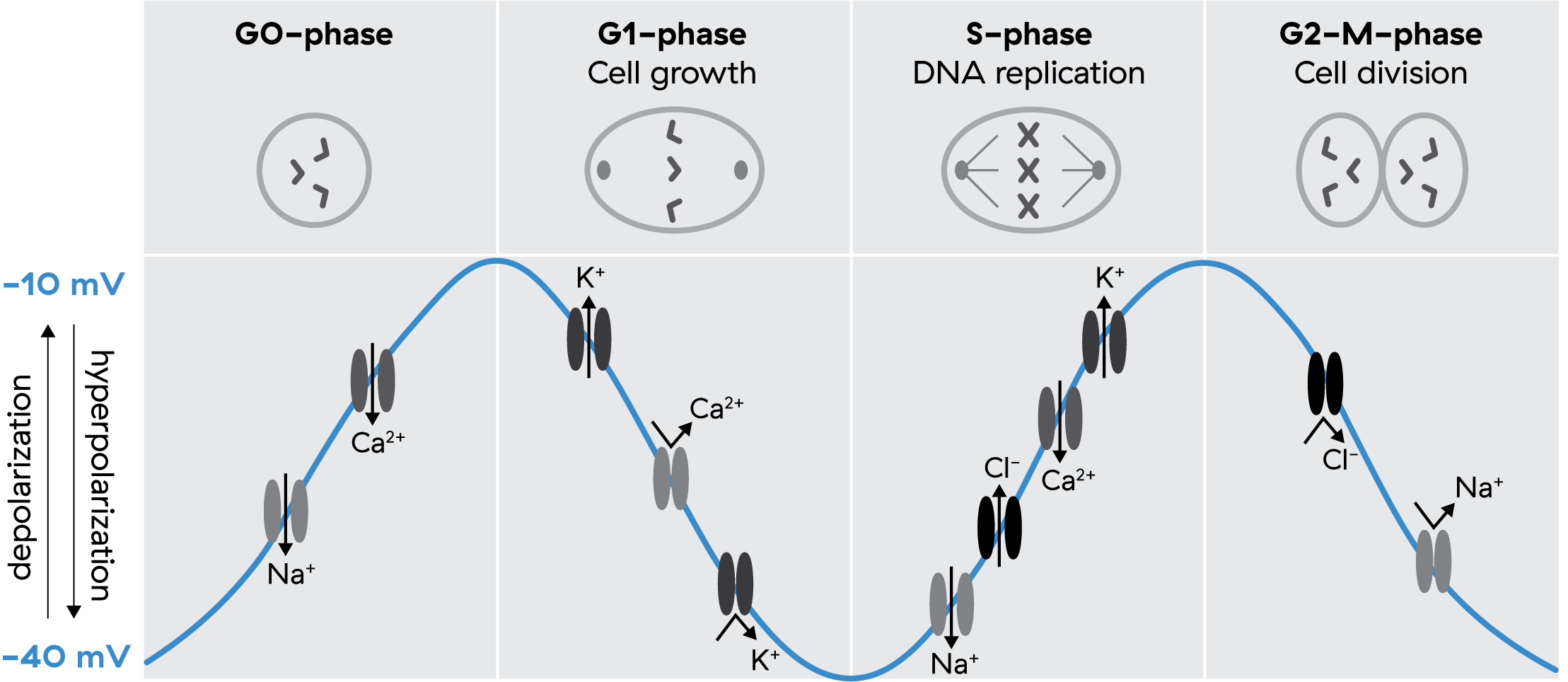
When we say ‘bioelectricity,’ we’re referring to changes in the concentrations of ions like sodium (Na+), potassium (K+), and calcium (Ca2+). These three ions carry most of the electrical charge that determines cellular membrane potential. As these ions move in and out of cells, this changes the voltage of the cell itself in a more positively or negatively charged direction.
For example, a fibroblast, a connective tissue cell, has a resting or average membrane potential of ~40 millivolts(mV), but this membrane potential will shift as much as +/- 20mV over the course of minutes to hours. All cells in your body work the same way that neurons do, just on much slower timescales, firing electrical signals carried via these charged ions that allow the cells to communicate and coordinate towards shared goals.
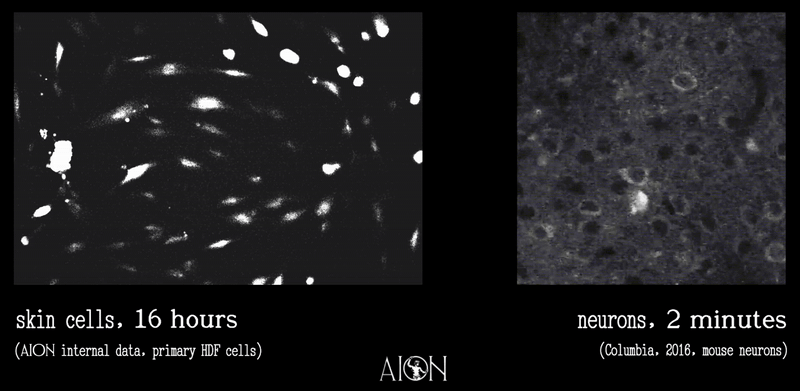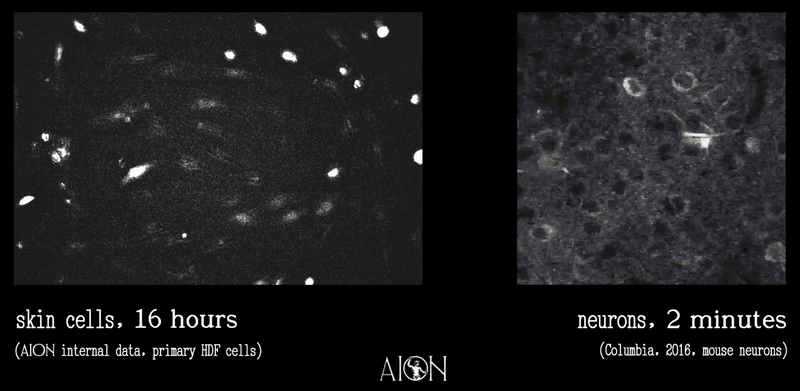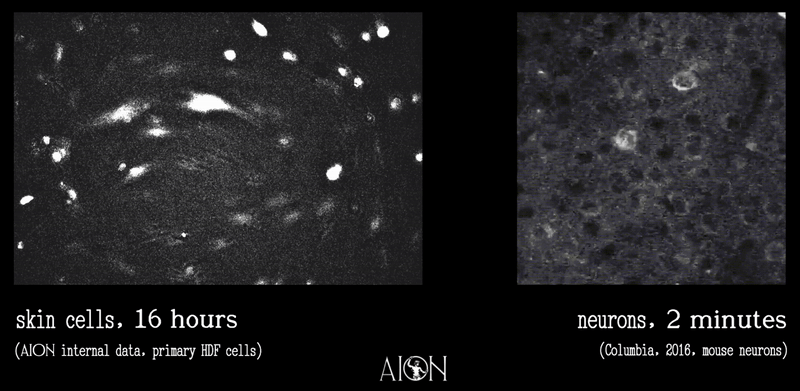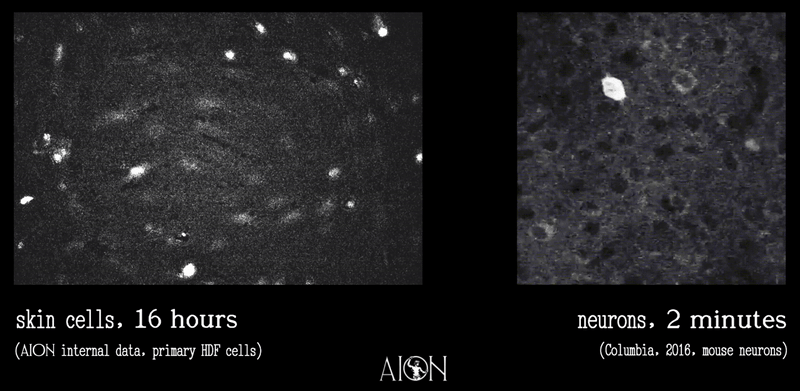
In looking at the membrane potential of a cell, it is sort of like looking at the temperature of a room. Because it is very hard to image all of the underlying thermodynamics in air that might give rise to, say, 70 degrees F, we can measure this single metric using simpler tools to infer underlying behaviors as much as is required to create control systems.
In the same vein, because it is very hard to see all the underlying ionic movements that give rise to a membrane potential of 70mV on a cell, we can look at just this to infer those movements well enough to create our control systems.
So why are we convinced that by changing the membrane potential at all that we can achieve highly effective control of cell states?
Starting with some historical examples from Clarence Cone, who uncovered the fundamental role bioelectricity plays in the cell cycle, here are some things we already know we can do with bioelectricity:
- Hyperpolarizing (more negative) halts division (e.g. in cancer) 6 7
- Depolarizing (less negative) triggers division in mature cells where it would otherwise not happen (e.g. mature neurons) 8
In addition to this, Cone found that cancer cells have a defunct bioelectric phenotype, which he hypothesized was a key contributor to driving unchecked growth.10 For example, myosarcomas maintained a -10 mV membrane potential compared to -90 mV in normal cells.
To what you’re thinking- yes, there is already proof in the present day market that these mechanisms can be exploited to address cancer. Novocure, a public company bringing in $600M+ per year in revenue, is already FDA approved and applying ‘Tumor Treating Fields’ as an adjunct to first-line chemotherapy in glioblastoma, with trials showing 5-month median survival improvement. The company is expanding into mesothelioma and pursuing over a dozen other cancer types.
Michael Levin is the modern champion of bioelectricity research. His lab has been able to carry forward the legacy of this work to achieve some incredible results. To name just a few:
- creating and reversing a melanoma11 12
- growing eyes where they shouldn’t be13
- regenerating limbs14
- reducing senescence15
- creating a two-headed worm through depolarization, directly combating gene-centric dogma for where body patterning information is contained16
Furthermore, Levin recently commented on X:
birth defects, failure to regenerate complex organs after damage, cancer, degenerative disease, and aging are all the same problem at root. It is all about how living matter implements a collective intelligence to maintain a specific anatomy over time (whether regenerating from: 1 egg cell, a.k.a. embryogenesis, from a damaged tissue, or from the small-scale wear and tear of adult life), and how we can facilitate that process of renewal. Regeneration, in the broadest sense, is the answer to all of these problems. It is not going to be possible to accelerate (or prevent, for those who want to) anti-aging research without feeding (or squelching) these other aspects of medicine and basic science. If you’re truly arguing against longevity research, it’s not just the elderly billionaires that you’re targeting, it’s also the kids with cancer, the people born with damaged organs, victims of injury, those damaged by pathogens, etc. etc. It’s all the same pool of suffering, with the same root cause.
For these reasons and more, we are convinced that exploiting the bioelectric interface is the key to solving aging. The problem remains: how do we achieve spatio-temporal control?
Neurostimulation Tools for Non-Neuronal Cells #
Because the above-mentioned mechanisms are more prevalent in neuroscience, this is also a space that cares a lot about solving this problem for achieving read-write control over the brain, and so here we find tools to draw inspiration for controlling non-excitable (no quick action potential) cells.
Neurostimulation stands as the most advanced field using external fields to modulate biology, alongside things like energy-based tumor ablation. FDA-approved therapies that entail applying fields with lasting effects include transcranial magnetic stimulation (TMS) for depression, OCD, smoking cessation, migraines and cranial electrotherapy (CES) for anxiety, insomnia and pain. This said, neurostimulation hits a few hard engineering walls because sub-millimeter precision is needed to be effective and fields must punch through skull and brain tissue that acts like a Faraday cage.
Shifting to translating these approaches to non-neuronal cells, required precision drops to centimeter scale since targets are bigger and, because there is no skull barrier, the ability to control the shape of ultrasound fields, referred to as beamforming, is simplified.
The advantage neurostimulation has is that, because of the action potential mechanism, neurons are able to rectify alternating current (AC) fields into direct current. So far for non-excitable cells, the field parameters to depolarize or hyperpolarize cells remains unknown. This is where AION is stepping in to map them first.
The challenge is that fields face a core trade-off between how precisely we can apply certain parameters in a given area with how deeply we can penetrate the body’s tissue. Light is able to focus very precisely while having very poor penetration depth, while on the flip side, magnetic fields are able to permeate through the body very freely with the challenge of focusing desired parameters over a given area.
That said, this trade-off narrows our search space dramatically. To penetrate deep tissue at a centimeter scale of precision, we can derive from first principles the properties of fields that will overcome this challenge in order to make our exploration feasible, and not something that costs billions of dollars over decades to find.
Traditional biochemistry does not offer us much of a jumping off point for which field parameters will do what we want in tissues. That’s where bioelectricity gives us the lens we need with the help of broader neuroscience findings which give us mechanisms of action: mechanical waves like ultrasound affect Piezo channels while electromagnetic fields trigger voltage-gated ion channels. Both of these are specialized proteins existing in the cell’s membrane that, when conformationally changed, alter the concentration of ions that are able to move between the inside and the outside of the cell, affecting its charge.
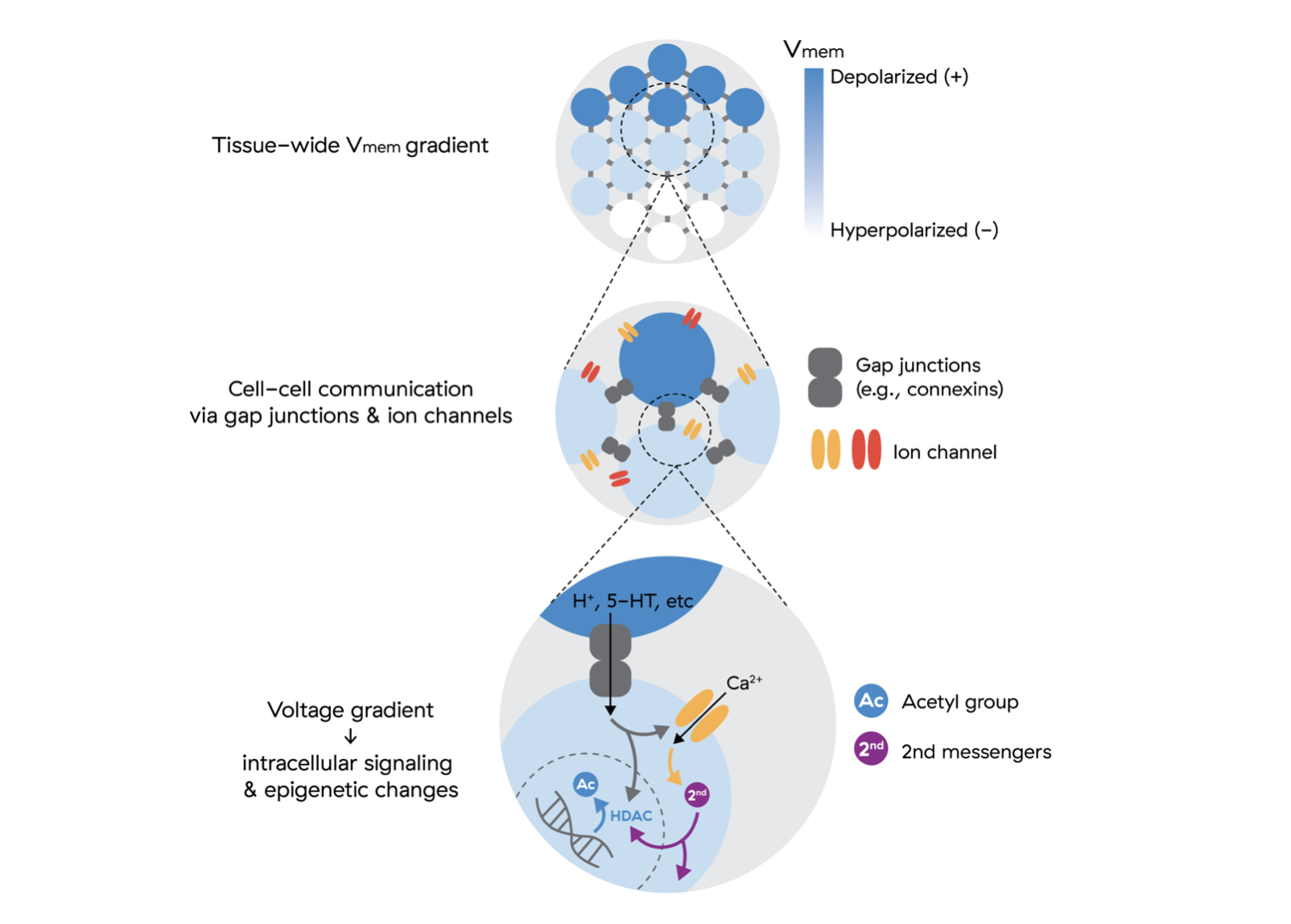
Levin has a quip: swap ’neuron’ for ‘cell’ and ‘milliseconds’ for ‘minutes’ in any neuroscience paper and you get to a developmental biology insight. Reprogramming cells mirror development in reverse, so external fields altering membrane potential should play a strong role in affecting the rates of reprogramming and differentiation.
This has already been confirmed through work that has shown to reprogram fibroblasts to stem cells by creating a temporary pore in the membrane via ultrasound.17
So, we know that hyperpolarizing cells halts division and that depolarizing them accelerates proliferation. Our hypothesis is that instead of relying on these blunt pushes in one direction or the other, by unlocking temporal control in this pattern of change over time, we can unlock the ability to effectively control a much more complex set of tissue states in real time.
In order to do this, we need to be able to very precisely obtain this information in the body in real time so that we can steer these patterns towards desired states inside an agent controlled feedback loop.
Here we find another core challenge in bioelectricity: how do we image patterns in deep tissue? Current imaging modalities for this work don’t scale beyond cell cultures or would be highly invasive in the body.
MRI as a Bioelectric Mapping Device #
We believe this is a solved problem with MRI. From the very start, the MRI was a bioelectricity mapping device.
The MRI was originally created by Raymond Damadian to be a machine that could differentiate cancer tissue from healthy tissue based on a realization that electrical properties of the cells correlated with water dynamics.
Damadian, in his 1971 paper proposing the MRI, stated “My own experiments with Escherichia coli (6) suggested that altered selectivity coefficients of alkali cations in biologic tissue, such as occur in neoplastic tissue (5), can indicate alterations in tissue water structure.”18 Here, alkali cations are sodium and potassium, the prime charge carriers in bioelectricity mentioned above, and tissue water structure changes are observable by the proposed MRI device.
In 2025, it was published that T2 times, a parameter that allows the MRI to have contrast between tissues based on water dynamics, correlate with experimentally altered membrane voltage, further validating this perspective.19 It makes sense. All biophysical parameters are inherently linked as they are different lenses through which to view the same whole. What matters to us is identifying properties we can measure in real time to build closed-loop control systems that apply fields to cells while simultaneously monitoring their state changes and adjusting the field parameters accordingly.
Bioelectromagnetics Literature #
For full cell fate control, we need to be able to remotely change membrane potential in both the positive and negative direction. Positive depolarization is easier. Bombarding the cell with the right mechanical or electrical fields cavitates the membrane through the above mentioned mechanisms, causing a leak in potassium and an influx of sodium. Mechanisms to increase the negative charge are more elusive, although we’ve found promising directions in the field studying risks from radio frequencies and common electronics with some of these studies having been interventional.
We will highlight two that we are exploring. The first is ion cyclotron resonance (ICR), first proposed by Abraham Liboff, and the second is magnetoacoustics.
ICR hypothesizes that ions resonate at specific frequencies in changing plus unchanging magnetic fields.20 The proposed mechanism here is the Lorentz force, however this is a flawed view given that the ion orbital path is too large to explain the effects seen in biology.21 That said, the data holds and has been shown to robustly replicate that there are specific frequency and field strength ratios, centered around ICR frequencies, that induce ion flows in living cells that can induce hyperpolarization.
In 2019, the National-Regional Key Technology Engineering Laboratory for Medical Ultrasound in Shenzhen showed that they could hyperpolarize cells in as little as 10 minutes by coupling a magnetic field with ultrasound.22 The proposed model is that the acceleration of ions through the magnetic field with ultrasound induces an electric field, but this lacks an explanation for how the created field affects the cell’s overall membrane potential because of the non-excitable cell’s missing action potential mechanism. It is possible that it is an artifact of the chamber design or that something more subtle than what fits into current biology models is happening at the membrane.
Despite encouraging data, replication is difficult in bioelectromagnetics and proposed physical models are messy and often wrong.
Mechanistic ambiguity is not a significant roadblock for us. Using closed-loop feedback, we can be mechanism-agnostic and let an agent determine optimal field parameters. Regardless, we still aim to benefit from previous mechanistic work by robustly replicating successes and testing boundaries of previous hypotheses. The record of success in the literature, even if physical models are wrong, is encouraging for our discovery prospects.
We are actively working to replicate these studies with field parameters that will be directly translatable to working in human tissues in the body. This leads to my closing comments on what we see as our most promising initial market.
Recapping what we know:
- Cone proved bioelectricity can control proliferation
- Novocure applies this to slow cancer growth via fields, likely altering cancer’s membrane potential
- Levin shows bioelectricity can robustly control differentiation and dedifferentiation
All of these are findings that, if exploited via fields, could be directly applied to the thymus to create massive value.
Thymus as Initial Target Market #
We are excited by the thymus as an initial target because, other than the central nervous system, it is arguable that there is no single organ that if rejuvenated would lead to longer, healthier lifespans in humans than the thymus. Everyone on the other side of maturity (20-30) starts to experience immune system decline as a product of aging-related changes in the thymus. By age 50-60, the thymus loses ~95% of its mass and reduces naïve T-cell production by over 90%.
The cruelest irony in oncology is that when chemotherapy works, then your immune system collapses. Globally, ~20M new cancer cases occur each year, with about half receiving chemotherapy. Severe immunosuppression as the direct result of thymic damage affects roughly a third of these patients. That’s ~3M people annually who survive cancer only to face life-threatening immune dysfunction, with no good solution beyond G-CSF regimens that don’t address the underlying thymic damage.
At pricing comparable to G-CSF ($25K+ per course), the immediate oncology TAM is roughly $80B. Even priced more accessibly, the market remains substantial.
The FDA endpoints for thymus-directed therapies are organ size and T-cell counts. Thymic function decline follows a defined trajectory in which thymic epithelial cells (TECs) transition into fibroblasts and subsequently adipocytes. Each step along this trajectory presents a potential point of intervention for our solution: doing virtually anything to cell fate can be useful in a thymic context.
The thymus is a perfect entry point because it is the first step towards our longer-term goal of bringing aging under complete biomedical control. Beyond cancer recovery, age-related thymic involution is one of the primary drivers of immune decline, autoimmunity, and infection risk. A therapy that restores thymic output has applications not only in oncology but also in extending healthspan and lifespan across aging populations.
For the thymus, the flagship device will be a smaller version of what can be thought of as a “read & write MRI,” as in, a device that images bioelectricity of the thymus while applying fields. Since the thymus is located in a small part of the upper chest, portable MRIs that focus on a small part of the body represent a likely form factor (e.g., similar to Hyperfine’s Swoop).
In realizing this vision, we will have crossed most of the unknown territory required in order to get us to the platonic idea of our vision, realised: a medbed in every home.
Med-Pod Concept from Elysium (2013)
Thank you Justin Mares, Sean Thiessen, Josh Noah, Eric Ward, Stephen Matic & Gabriel Anderson for feedback on this write-up.
#
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663-76. doi: 10.1016/j.cell.2006.07.024. Epub 2006 Aug 10. PMID: 16904174. ↩︎
Guan, J., Wang, G., Wang, J. et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 605, 325–331 (2022). https://doi.org/10.1038/s41586-022-04593-5 ↩︎
If you want to make every permutation of a 300 amino acid protein, to do that you would need more atoms than exist in the known universe. The human genome has about 1,600 transcription factor proteins of which 4 are the Yamanaka Factors. Even for a simple cocktail of just 4 factors, the possible combinations top 272 billion. For mixes of 7 or 10, the numbers explode to around 5 × 10^18 or 3 × 10^25, way beyond what labs can test in a lifetime. Assume as an overestimation that labs screen 100,000 inputs a year. Over 20 years, that’s about 2 million tests. For that basic 4-factor case, they’ve covered a fraction of just 0.0007%. On the flip side, for a cocktail of just 4 chemicals, the possible combinations number over 40 sextillion. That’s a 4 followed by 22 zeros. For mixes of 7 or 10, the numbers explode to levels like 10^41 or 10^53, way beyond what we can count in a lifetime. For simplicity, assume again companies screen 100,000 compounds a year. Over 20 years, that’s about 2 million tests. For that basic 4-chemical case, they’ve covered a fraction so tiny it’s like checking one atom in the entire mass of Earth. For bigger mixes, consider the 27 chemicals used in the cocktails above, it’s basically zero. Brute-force testing can’t touch this huge space, no matter the billions spent. Real progress needs to be driven by a better idea. That is where we come in. ↩︎
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JL, Xu J, Rodriguez Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua Belmonte JC. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell. 2016 Dec 15;167(7):1719-1733.e12. doi: 10.1016/j.cell.2016.11.052. PMID: 27984723; PMCID: PMC5679279. ↩︎
Parras, A., Vílchez-Acosta, A., Desdín-Micó, G. et al. In vivo reprogramming leads to premature death linked to hepatic and intestinal failure. Nat Aging 3, 1509–1520 (2023). https://doi.org/10.1038/s43587-023-00528-5 ↩︎
Clarence D. Cone, Jr.; Variation of the Transmembrane Potential Level as a Basic Mechanism of Mitosis Control. Oncology 1 June 1970; 24 (6): 438–470. https://doi.org/10.1159/000224545 ↩︎
Cone, C. D. (1971). Unified theory on the basic mechanism of normal mitotic control and oncogenesis. Journal of Theoretical Biology, 30(1), 151–181. https://doi.org/10.1016/0022-5193(71)90042-7 ↩︎
Clarence D. Cone, Jr., Charlotte M. Cone ,Induction of Mitosis in Mature Neurons in Central Nervous System by Sustained Depolarization.Science192,155-158(1976).DOI:10.1126/science.56781 ↩︎
Anderson, B. (2023). Bioelectricity: A top-down control model to promote more effective aging interventions. Bioelectricity, 00(00), 1-11. https://doi.org/10.1089/bioe.2023.0013 ↩︎ ↩︎
Cone, C. D. (1971). Unified theory on the basic mechanism of normal mitotic control and oncogenesis. Journal of Theoretical Biology, 30(1), 151–181. https://doi.org/10.1016/0022-5193(71)90042-7 ↩︎
Lobo, D., Lobikin, M. & Levin, M. Discovering novel phenotypes with automatically inferred dynamic models: a partial melanocyte conversion in Xenopus. Sci Rep 7, 41339 (2017). https://doi.org/10.1038/srep41339 ↩︎
Chernet B. T., Levin M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget. 2014; 5: 3287-3306. Retrieved from https://www.oncotarget.com/article/1935/text/ ↩︎
Vaibhav P. Pai, Sherry Aw, Tal Shomrat, Joan M. Lemire, Michael Levin; Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 15 January 2012; 139 (2): 313–323. doi: https://doi.org/10.1242/dev.073759 ↩︎
Nirosha J. Murugan et al. ,Acute multidrug delivery via a wearable bioreactor facilitates long-term limb regeneration and functional recovery in adult Xenopus laevis.Sci. Adv.8,eabj2164(2022).DOI:10.1126/sciadv.abj2164 ↩︎
Assistant: Sediqi, H., & Levin, M. (2025). Bioelectric characterization of senescing human keratinocytes. iScience, 28(9), 113275. https://doi.org/10.1016/j.isci.2025.113275 ↩︎
Beane WS, Morokuma J, Adams DS, Levin M. A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol. 2011 Jan 28;18(1):77-89. doi: 10.1016/j.chembiol.2010.11.012. PMID: 21276941; PMCID: PMC3278711. ↩︎
Lee, Y. S., Heo, H., Lee, J., Moon, S. U., Jung, W. Y., Park, Y. K., Park, M. G., Oh, S.-H., & Kim, S. (2017). An ultra-effective method of generating extramultipotent cells from human fibroblasts by ultrasound. Biomaterials, 143, 65–78. https://doi.org/10.1016/j.biomaterials.2017.07.033 ↩︎
Raymond Damadian ,Tumor Detection by Nuclear Magnetic Resonance.Science171,1151-1153(1971).DOI:10.1126/science.171.3976.1151 ↩︎
Kyeongseon Min, Sungkwon Chung, Seung-Kyun Lee, Jongho Lee, Phan Tan Toi, Daehong Kim, Jung Seung Lee, Jang-Yeon Park (2025) Responses to membrane potential-modulating ionic solutions measured by magnetic resonance imaging of cultured cells and in vivo rat cortex eLife 13:RP101642 https://doi.org/10.7554/eLife.101642.3 ↩︎
Liboff, Abraham R.. “Ion Cyclotron Resonance interactions in living systems.” (2013). ↩︎
Binhi, V. N., & Goldman, R. J. (2000). Ion–protein dissociation predicts ‘windows’ in electric field-induced wound-cell proliferation. Biochimica et Biophysica Acta (BBA) - General Subjects, 1474(2), 147–156. https://doi.org/10.1016/S0304-4165(00)00002-7 ↩︎
Hu Y, Wang Y, Chen X, Chen S. Sonomagnetic Stimulation of Live Cells: Electrophysiologic, Biochemical and Behavioral Responses. Ultrasound Med Biol. 2019 Nov;45(11):2970-2983. doi: 10.1016/j.ultrasmedbio.2019.07.009. Epub 2019 Aug 13. PMID: 31416657. ↩︎